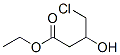Product Description
ETHYLL-4-CHLORO-3-HYDROXY BUTYRATE CAS number is 10488-69-4,It is an important organic intermediate, Its main use is to synthesize precursor compounds of cholesterol-lowering drugs, but also for many activities Synthesis of drugs such as hydroxymethylglutaryl reductase inhibitors and 1,4-dihydropyridine β-blockers. ETHYLL-4-CHLORO-3-HYDROXY BUTYRATE has the advantage of being easy to synthesize and inexpensive, and it is a very cost-effective way to prepare ETHYLL-4-CHLORO-3-HYDROXY BUTYRATE by asymmetric reduction as a reaction raw material. Moreover, during the reaction, the microorganism does not use the product of the reaction to produce other new impurities, so that the preparation reaction can be carried out by using whole cells of the microorganism, and the reaction method is simpler.
At present, there have been many reports on the preparation of chiral ETHYLL-4-CHLORO-3-HYDROXY BUTYRATE by asymmetric reduction of ethyl 4-chloroacetoacetate. The main preparation methods are chemical methods and biological methods. Among them, microbial catalysis in biological methods is a hot spot of research. Microbial catalysis utilizes the stereoselectivity of a specific microbial cell-specific enzyme system to selectively convert a substrate into a product in a cell. However, due to the complexity of the enzyme system present in the cell, the optical purity of the catalytic product is not high, and it is necessary to screen an excellent microbial strain or to improve by cloning a recombinant gene. Coenzyme addition is also required to provide the reducing power of the reaction.
In the technique of preparing DL-4-chloro-3-hydroxybutyric acid ethyl ester by asymmetric reduction of ethyl 4-chloroacetoacetate by microbial catalytic method, the substrate conversion rate is low, the optical purity of the product is low, and the coenzyme cycle regeneration is solved. Need to be further studied and resolved. Someone specially designed a technical solution for this: using 4-chloroacetoacetate as a substrate, glucose as a substrate, NAD(P)+ as a cofactor, and introducing a ketoreductase gene and glucose dehydrogenation. Recombinant Escherichia coli with the enzyme GDH gene was subjected to a transformation reaction to prepare an asymmetric solution of Ethyll-4-Chloro-3-Hydroxy Butyrate. This method effectively solves various major defects in the asymmetric reduction of Ethyll-4-Chloro-3-Hydroxy Butyrate CAS 10488-69-4 microbial catalytic method.
Thera. Category: Organic intermediate
Cas No.:10488-69-4
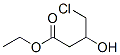
Synonym:COBE;ETHYLL-4-CHLORO-3-HYDROXY BUTYRATE;Ethyl-4-Chloro-4-HydroxyButyrate;
Molecular Formula:C6H11ClO3
Molecular Weight:166.60274
Assay: ≥99.%
Packing: Export worthy packing
lMaterial Safety Data Sheet: Available on request

.png) Contact Now
Contact Now