Product Description
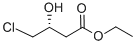
Ethyl (R)-(+)-4-chloro-3-hydroxybutyrate CAS number 90866-33-4, which is an important chiral building block for the synthesis of (R)-γ-amino-β- Butyric acid, L-carnitine and (-)-large lactam can also be converted to a synthon of (+)-neomycin and chiral 2,5-cyclohexadienone. The method for preparing optically chiral Ethyl (R)-(+)-4-chloro-3-hydroxybutyrate can be classified into a chemical catalytic hydrogenation method and a biocatalytic method. Among them, the chemical catalytic hydrogenation method uses 4-chloroacetoacetate as a reaction precursor and a rare metal ruthenium complex as a catalyst for asymmetric catalytic hydrogenation. The advantage of this method is that it is suitable for large-scale production, but This method has the disadvantages of high catalyst cost, unfriendly environment and unsatisfactory optical purity of the product, and requires high pressure in the catalytic process, so the equipment requirements are also high. The biocatalytic method has the advantages of high activity, high stereoselectivity, mild reaction conditions and good social benefits. Therefore, the preparation of Ethyl (R)-(+)-4-chloro-3-hydroxybutyrate CAS number 90866-33-4 by biocatalyst is highly favored by researchers.
Biocatalytic asymmetric reduction of Ethyl (R)-(+)-4-chloro-3-hydroxybutyrate CAS number 90866-33-4 is mainly carried out under the catalytic action of dehydrogenase or carbonyl reductase, and also requires the reduction of coenzyme NADH or NADPH. participate. However, the coenzyme NADH or NADPH is expensive and therefore limits its application. Coenzyme NADH or NADPH regeneration and circulation can be economically achieved using a coenzyme circulation system throughout the cell (Wang L. J., et al. Bioresour. Technol. 2011, 102, 7023-7028). The chiral purity of the final product catalyzed by carbonyl reductase reported in some literature is only about 99%, mainly due to the lower concentration of its catalytic substrate 4-chloroacetoacetate and usually needs to be divided during the reaction. Batch feed, or add expensive NADH or NADPH to achieve a satisfactory substrate concentration. And most of the literature reports about the biosynthesis of (S)-4-chloro-3-hydroxybutyrate. Therefore, it is imperative to find a highly active and selective strain to reduce ethyl 4-chloroacetoacetate to synthesizeEthyl (R)-(+)-4-chloro-3-hydroxybutyrate CAS 90866-33-4.
A research team constructed a recombinant reductase strain, Escherichia coli, using genome engineering. High-efficiency synthesis of (R)-(+)-4-chloro-3-hydroxybutyric acid ethyl ester can be achieved by using its whole-cell catalyst with glucose as a secondary substrate and low-cost NAD+ to achieve coenzyme regeneration. No batch feeding is required during the reaction, and in the 2 L reaction system, the substrate concentration can reach 800-6000 mM, The rate of Ethyl (R)-(+)-4-chloro-3-hydroxybutyrate CAS number 90866-33-4 can be as high as 99.0%. Therefore, it has potential application prospects.
Thera. Category: Organic intermediate
Cas No.:90866-33-4
Synonym: (R)-ETHYL 4-CHLORO-3-HYDROXYBUTYRATE;(R)-(+)-4-CHLORO-3-HYDROXYBUTYRIC ACID ETHYL ESTER;(R)-4-CHLORO-3-HYDROXYBUTYRIC ACID ETHYL ESTER
Molecular Formula:C6H11ClO3
Molecular Weight:166.6
Assay: ≥99.%
Packing: Export worthy packing
lMaterial Safety Data Sheet: Available on request

.png) Contact Now
Contact Now
