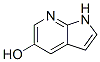Product Description
Using 5-bromo-7-azaindole as raw material, protected by amino group, hydrolyzed by boric acid at low temperature to obtain hydroxy substituent,The starting material of the route is expensive; and it requires the use of lithium bis-trimethylsilylamine, butyl lithium, and the amplification operation is unsafe; Using 5-bromo-7-azaindole as a raw material, replacing bromine with sodium methoxide, and then demethoxylating to obtain a hydroxyl group,The route is also expensive in starting materials; the amount of sodium methoxide is too large, and the post-treatment produces a lot of wastewater; in addition, boron tribromide is easy to volatilize and has high toxicity, which is not convenient for industrial scale-up operation;using 5-bromo-7-azaindole as a raw material,The 5-hydroxy-7-azaindole CAS number 98549-88-3 of formula III is finally obtained two steps by nitrogen protection, hydroxylation and direct deprotection.
400 ml of tetrahydrofuran was added to a 1 L three-necked flask at room temperature to start stirring. Add 5-bromo-7-azaindole (30 g, 0.15 mol) and potassium t-butoxide (25.6 g, 0.23 mol) to the reaction flask, stir and dissolve, and then cool the ice salt bath until the internal temperature is 0℃. Triisopropylchlorosilane (30.8 g, 0.16 mol) diluted with 80 ml of tetrahydrofuran was added dropwise. After the addition is completed, the reaction is maintained at 0-5℃ for 10-20 minutes. TLC is controlled and the reaction is over. 300 ml of water was added to the reaction flask, and the mixture was stirred for 15 minutes, and the liquid phase was separated. The aqueous phase was extracted with methyl tert-butyl ether (200 ml*2), the organic phase was combined, and the organic phase was washed with 500 ml of a saturated aqueous solution of sodium chloride. After drying over anhydrous sodium sulfate, the solvent was concentrated to give an oily substance, 50 ml of methanol was added, and a solid was precipitated, and dried in a vacuum vacuum oven to obtain a compound of the formula II 5-bromo-1-(triisopropylsilyl)-pyrrolo[2,3-b]pyridine
A compound of formula II (4.08 g, 11.5 mmol), copper acetylacetonate (0.6 g, 2.3 mmol), BHMPO (0.76 g, 2.3 mmol), lithium hydroxide monohydrate (1.7) was added to a 50 ml reaction flask. g, 40 mmol), 18 ml of DMSO, 4.5 ml of water, stirring was started, replaced with nitrogen, and the temperature was raised to an internal temperature of 130℃, and the temperature was maintained for 1 hour.The reaction of the medium-controlled raw material is finished, the heating is stopped, the temperature is lowered to room temperature (20-30℃), 100 ml of water is added, the pH is adjusted to 6 with 2N diluted hydrochloric acid, a solid precipitates, and suction filtration is carried out, and the aqueous phase is extracted with ethyl acetate 50 ml*3, and combined. The organic phase is washed once with water and a saturated aqueous solution of sodium chloride, dried over anhydrous sodium sulfate and concentrated to give compound of formula III 5-hydroxy-7-azaindole CAS number 98549-88-3(ABT199 Intermediate).
Thera. Category: Leukemia Drugs
Cas No.: 98549-88-3
Synonyms: 2-(4-Chlorophenyl)-4,4-dimethyl-1-cyclohexene-1-carbaldehyde;2-(4-Chlorophenyl)-4,4-dimethylcyclohex-1-enecarboxaldehyde;2-(4-chlorophenyl)-4,4-dimethylcyclohex-1-enecarbaldehyde;4'-chloro-5,5-diMethyl-3,4,5,6-tetrahydro-[1,1'-biphenyl]-2-carbaldehyde;2-(4-chlorophenyl)-4,4-dimethyl-1-cyclohexene-1-carboxaldehyde;
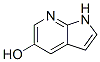
Molecular Weight: 134.14
Purity: ≥98%
Packing: Export worthy packing
Material Safety Data Sheet: Available on request
|
ABT-199 intermediates
|
|
2-(4-chlorophenyl)-4,4-dimethyl-1-Cyclohexene-1-carboxaldehyde
|
1228837-05-5
|
|
(2-(4-chlorophenyl)-4,4-diMethylcyclohex-1-enyl)Methanol
|
1228780-51-5
|
|
1-[[2-(4-chlorophenyl)-4,4-dimethyl-1-cyclohexen-1-yl]
methyl]-Piperazine, hydrochloride (1:2)
|
1628047-87-9
|
|
4-Aminotetrahydro-4H-pyran
|
130290-79-8
|
|
3-nitro-4-((tetrahydro-2H-pyran-4-yl)MethylaMino)
benzenesulfonaMide
|
1228779-96-1
|

.png) Contact Now
Contact Now