Product Description
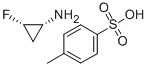
SITAFLOXACIN intermediate also known (1R,2S)-FLUOROCYCLOPROPYLAMINE TOSYLATE CAS number 143062-84-4, in the "China Pharmaceutical Industry magazine" published in 2006 on the 36th page recorded the "sitafloxacin synthesis route diagram" and described a series of synthetic methods of sitafloxacin, which lists a variety of methods for the preparation of (1R,2S)-FLUOROCYCLOPROPYLAMINE TOSYLATE . Since it is a chiral molecule, it is easy to appear antiisomeric isomers in the synthesis process, during the separation of the isomers not only the overall yield level is not high, the more critical is the need for separation using column chromatography, which became the bottleneck of large-scale production. Through the long-term practice, we have explored a new method of preparation of (1R,2S)-FLUOROCYCLOPROPYLAMINE TOSYLATE, pls see the following detail steps: 1. Construction of ternary ring: dissolve dimethyl malonate and 1,1,2-tribromo- 2-fluoroethane in anhydrous dimethylformamide, anhydrous potassium carbonate is added in portions and heated in a water bath at 25-35 ° C to give a solution to dry the solvent to give the first compound. 2. Dehydrogenation: dissolve the first step product in methanol, palladium on carbon as a catalyst through the hydrogen reaction to obtain the second liquid oily compounds. 3. To branch chain:dissolve the oily liquid in dimethyl formamide and salt water mixture to get the third step compound. 4. Hydrolysis. 5. Chiral split. 6. restore. 7. Introduction of amino groups. 8. Substitution: dissolve the seventh step of the introduction of amino compounds and p-toluenesulfonic acid in acetonitrile, stirring reaction 36-40 hours room temperature, concentrated to get (1R,2S)-FLUOROCYCLOPROPYLAMINE TOSYLATE CAS number 143062-84-4.
Thera. Category: Antibacterial
Cas No.: 143062-84-4
Synonyms: CarbaMic acid, N-[(7S)-5-(phenylMethyl)-5-azaspiro[2.4]hept-7-yl]-, 1,1-diMethylethyl ester;(S)-tert-Butyl (5-benzyl-5-azaspiro[2.4]heptan-7-yl)carbaMate;[(7S)-5-(Phenylmethyl)-5-azaspiro[2.4]hept-7-yl]carbamic;Carbamic acid, [(7S)-5-(phenylmethyl)-5-azaspiro[2.4]hept-7-yl]-, 1,1-dimethylethyl ester;[(7S)-5-(Phenylmethyl)-5-azaspiro[2.4]hept-7-yl]carbamic acid tert-butyl ester;
Molecure Structure:C10H14FNO3S
NW: 302.41124
Assay: ≥99%
Packing:Export worthy packing
Material Safety Data Sheet:Available on request
Usage: Use to making Sitafloxacin which is a fluoroquinolone antibiotic that shows promise in the treatment of Buruli ulcer.
Related intermediates:
1) (S)-7-tert-Butoxycarbonylamino-5-azaspiro[2.4]heptane CAS number 127199-45-5
2) Carbamic acid, [(7S)-5-(phenylmethyl)-5-azaspiro[2.4]hept-7-yl]-, 1,1-dimethylethyl ester CAS 144282-37-1
3) (1R,2S)-FLUOROCYCLOPROPYLAMINE TOSYLATE CAS 143062-84-4

.png) Contact Now
Contact Now
