Product Description
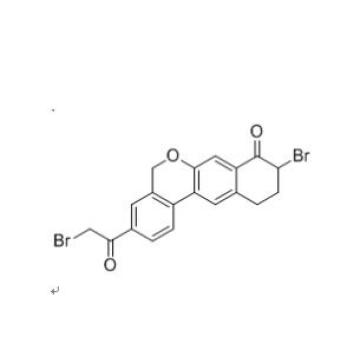
Velpatasvir intermediate 9-Bromo-3-(2-Bromo Acetyl)-10,11-Dihydro-5H-dibenzo(c,g) Chromen-8(9H)-one CAS number is 1438383-89-1, his synthesis process is as following:
1. Dissolve 5.0 g of 2-bromo-5-iodobenzyl alcohol in dry 40 mL of tetrahydrofuran, cool the system to below -10 ° C, and slowly add 17.6 mL of isopropylmagnesium chloride to the reaction temperature to control the reaction temperature not to exceed -10 ° C. After completion of the dropwise addition, the reaction was stirred for 1 hour with stirring, and then 3.73 mL of N-methoxy-N-methylacetamide was added dropwise thereto. After 1 h, the reaction solution was heated to 20 ° C, then the reaction solution was cooled to about 0 ° C, and quenched with 25 mL of 3N hydrochloric acid. The reaction mixture was extracted with 50 mL of methyl tert-butyl ether. The organic layer was sequentially taken with 50 mL of 1 M HCl, 50 mL The organic layer was washed with water and evaporated to dryness to dryness crystals.
2. Dissolve 1-(4-bromo-3-(hydroxymethyl)phenyl)ethanone in 30 mL of tetrahydrofuran, and then add 2.82 mL of triethylamine thereto. After the addition, the reaction system was cooled to 0 ° C and stirred. 1.15 mL of methanesulfonyl chloride was added dropwise, and the reaction was incubated at 0 ° C for 30 min, then 2.9 g of anhydrous lithium chloride was added thereto, and the reaction was stirred at room temperature for 2 h. After completion of the reaction, 30 mL of methyl t-butyl ether and 15 mL were added thereto. The organic layer was washed with 15 mL of water, dried over anhydrous sodium sulfate, and dried, and then the mixture was evaporated to dryness, and the residue was added to 15 ml of n-heptane for 2 h to precipitate a solid, which was filtered and washed with 5 mL of n-heptane to get yellow solid 1-(4-bromo-3-(chloromethyl)phenyl)ethanone 2.43 g.
3. Add 6.0 g of 1-(4-bromo-3-(chloromethyl)phenyl)ethanone, 3.93 g of 7-hydroxytetralinone, 6.68 g of potassium carbonate and 0.78 g of tetrabutylammonium bromide to the reaction flask. Then, 30 mL of N,N-dimethylacetamide was added thereto, and the reaction was stirred at room temperature for 20 hours. After the reaction was completed, 180 mL of ethyl acetate and 60 mL of water were added to the reaction system, and the layers were separated, and the organic layer was washed with 60 mL of water. After drying over anhydrous sodium sulfate, the organic layer was concentrated under reduced pressure to about 50 mL, and concentrated under reduced pressure. The mixture was evaporated to room temperature and filtered, and the filter cake was washed with 20 mL of ethyl acetate.
4. Add 50 g of the brownish yellow solid obtained in the step 3, 205 mg of pivalic acid, 175.5 mg of triphenylphosphine, 150 mg of palladium acetate, and 1.02 g of potassium carbonate to 50 mL of N,N-dimethylacetamide. After the replacement, the reaction was carried out at 80 ° C for 5 h under nitrogen atmosphere. After the reaction was completed, the reaction solution was cooled to room temperature, 50 mL of ethyl acetate and 75 mL of water were added thereto, and the layers were separated, and the organic layer was washed with 25 mL of water and dried over anhydrous sodium sulfate. The solution was concentrated under reduced pressure to a volume of ca. 10 mL, and then concentrated under reduced pressure. The mixture was evaporated to room temperature, filtered, and then filtered and washed with 5 mL of EtOAc.
5. Add 1.0 g of the yellow solid obtained in the step 4 to 20 mL of a 9:1 dichloromethane-methanol mixed solvent, and add 2.5 g of tribromopyridinium salt thereto with stirring, and add the reaction at room temperature for 5 hours, complete the reaction, filter, and filter. The cake was washed with 10 mL of methanol to give 1.13 g of a yellow solid, which was the Velpatasvir intermediate 9-Bromo-3-(2-Bromo Acetyl)-10,11-Dihydro-5H-dibenzo(c,g) Chromen-8(9H)-one CAS number 1438383-89-1.
Velpatasvir intermediate named 9-Bromo-3-(2-Bromo Acetyl)-10,11-Dihydro-5H-dibenzo(c,g) Chromen-8(9H)-one CAS number is 1438383-89-1,Molecular formula is C19H14Br2O3 Molecular weight is (m / min) 450.12. We produce this intermediate in large quantities, with sufficient stock. We can provide sample testing free of charge. If the buyer confirms the order within 15 days after the sample inspection. We are committed to maintaining the quality of the sample in accordance with the quality of the bulk order, If the order is confirmed more than 15 days or longer, we will ensure that the HPLC test result between the sample and the batch is less than 0.3%.
Thera. Category: Anti Viral
Cas No.: 1438383-89-1
Synonym: 9-Bromo-3-(2-Bromo Acetyl)-10,11-Dihydro-5H-dibenzo(c,g) Chromen-8(9H)-one;9-Bromo-3-(2-bromoacetyl)-10,11-dihydro-5H-benzo[d]naphtho[2,3-b]pyran-8(9H)-one
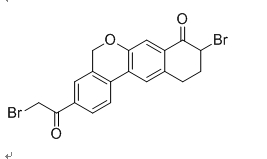
Molecular Formula: C19H14Br2O3
Molecular Weight: 450.12
Assay: ≥99.%
Solubility: Sparingly Soluble (1.2E-3 g/L) (25 ºC), Calc.*
Density: 1.514±0.06 g/cm3 (20 ºC 760 Torr), Calc.*
Packing: Export worthy packing
lMaterial Safety Data Sheet: Available on request
Usage:Velpatasvir Intermediate
Related Intermediate:
1) (2S,5S)-N-Boc-5-Methylpyrrolidine-2-Carboxylic Acid CAS 334769-80-1
2) Ethyl (2S,5S)-5-Methylpyrrolidine-2-Carboxylate CAS 676560-84-2
3) (5S)-N-(Methoxycarbonyl)-L-Valyl-5-Methyl-L-Proline CAS 1335316-40-9
4) 7-Hydroxy-1-Tetralone, MFCD01312225 CAS 22009-38-7
5) (R)-2-(methoxycarbonylamino)-2-phenylacetic acid CAS 50890-96-5
6) 9-Bromo-3-(2-Bromo Acetyl)-10,11-Dihydro-5H-dibenzo(c,g) Chromen-8(9H)-one CAS number is 1438383-89-1
7) 3-(2-bromoacetyl)-10,11-dihydro-5H-Benzo[d]naphtho[2,3-b]pyran-8(9H)-one CAS number 1378390-29-4,
8) GS-5816 interMediate, CAS NUMBER 1378388-16-9

.png) Contact Now
Contact Now
