Product Description
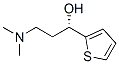
Duloxetine chiral intermediate (S)-(-)-N,N-Dimethyl-3-hydroxy-3-(2-thienyl)propanamine CAS number 132335-44-5, which is the key intermediate for the the synthesis of duloxetine. At present, most of them are obtained by chemical resolution. Some person use chiral acids as resolving agents to prepare this chiral intermediate of duloxetine. The main disadvantage is that chemical resolution can only generally obtain one of the enantiomers. The highest theoretical yield of chemical resolution is only 50%, which results in low utilization of raw materials, at least half of the isomers cannot be recycled, and the recycling of compounds is low, which does not meet the requirements of green chemistry and atomic economics. Asymmetric synthesis has also been used to prepare this chiral intermediate of duloxetine, which uses asymmetric catalysts to asymmetrically reduce the carbonyl to hydroxyl groups. The main disadvantage of this method is that the obtained chiral intermediate has low optical rotation, and Catalytic asymmetric hydrogenation reactions are more dangerous and require higher device equipment, making it difficult to scale up production. After a comprehensive comparison, we found that there is a reliable method. The specific process is as follows:
1. Dissolve 2-acetyl thiophene, dimethylamine hydrochloride and paraformaldehyde in isopropanol, add concentrated hydrochloric acid, reflux for 8 hours, cool to room temperature, filter by suction,use filter cake washed with cold ethanol, and dry to get White solid;
2. The obtained above white solid, ethanol and water were mixed and dissolved by stirring. NaOH was slowly added at room temperature and the pH was adjusted to 11-12. Then sodium borohydride was added and the mixture was reacted at room temperature overnight. After the reaction was completed, acetone was added to quench the reaction. Ethanol was distilled off under pressure and a white solid precipitated. It was filtered and dried to give a white solid.
3. Dissolve (S)-mandelic acid in ethanol at 50°C. The (S)-mandelic acid in ethanol solution was slowly added to the methyl tert-butyl ether of N,N-dimethyl-3-hydroxy-3-(2-thiophene)-propylamine as a white solid obtained in above Step 2. In the solution, white solids was continuously precipitated, the slurry was heated to reflux for 45 min, then stirred at room temperature for 1 h, filtered, and the filtrate was washed twice with water, dried and concentrated to give (R)-N,N-dimethyl-3- Hydroxy-3-(2-thiophene)-propylamine, the filter cake is (S)-N,N-dimethyl-3-hydroxy-3-(2-thiophene)-propylamine mandelate; wherein (S) The ratio of mandelic acid to ethanol is 0.21:50 mol/ml.
4. The (S)-N,N-dimethyl-3-hydroxy-3-(2-thiophene)-propylamine mandelate obtained in above Step 3 was dissolved in water and alkalized with 5N NaOH, precipitated a large amount of white solid.Then filtered, washed twice with water and dried to give a white solid. This white solid is the target compound (S)-(-)-N,N-Dimethyl-3-hydroxy-3-(2-thienyl)propanamine CAS number 132335-44-5
Thera. Category:Anti-Diabetes
Cas No. 132335-44-5
Synonyms:(S)-1-BETA-HYDROXY-1-(2-THIENYL)-3-DIMETHYLAMINOPROPANE;(s)-3-(dimethylamino)-1-(2-thienyl)-1-propanol;(S)-N,N-dimethyl-3-hydroxy-3-(2-thienyl)-2-propanamine;Duloxetine intermediate AA;(S)-(-)-N,N-dimethyl-3-hydroxy-3-(2-thienyl)propylamine;(S)-(-)-N-DIMETHYL-3-HYDROXY-3-(2-THIENYL)-PROPANAMINE;S-(-)-N,N-DIMETHYL-3-HYDROXY-3-(2-THIENYL)PROPANAMINE;(S)-1-HYDROXY-1-(2-THIENYL)-3-DIMETHYLAMINOPROPANE;
Molecular Formula: C9H15NOS
Molecular Weight: 185.29
Purity: ≥99%
Packing: Export worthy packing
Material Safety Data Sheet: Available on request

.png) Contact Now
Contact Now
