Product Description
Nintedanib side chain CAS number is 262368-30-9, it has been reported in the literature to use N-methyl-4-amino-nitrobenzene as raw material, with chloroacetyl chloride reaction to form amide, the treated amide and N-methyl piperazine Oxazine take reaction, after the reaction was completed and then hydrogenated to obtain the target product nintedanib side chain. The main problems of this synthesis method are as follows: (1) N-methyl-4-aminonitrobenzene is reacted with chloroacetyl chloride to form amide. The reaction is performed by heating the main raw material and the solvent to near reflux temperature, Chloroacetyl chloride was added dropwise. After the reaction with chloroacetyl chloride was completed, the temperature was lowered to 60℃, methyl cyclohexane was added as a poor solvent, and then re-cooled to 0℃,crystallized for 1 hour. The operation was cumbersome and the solvent was a mixed solvent,high cost, unable to Recycle. After got the amide, must be separated, purified, dried and other operations in order to carry out the next reaction, which greatly increased the production man-hours and energy consumption. (2) the amide obtained in the first step and solvent are heated to 40℃, then N-methylpiperazine is added dropwise and then warmed to 50℃ to react. After completion of the substitution reaction, isopropanol is further added and under Pd / C catalysis, Hydrogen reduction.add into isopropanol. Operation is more complicated.
In view of the above existing problems, some people have made adjustments to the method for synthesizing the side chain of Nintedanib CAS 262368-30-9 and prepared the one-pot method as follows: N-methyl-4-aminonitrobenzene is reacted with chloroacetyl chloride in a solvent, Then washing the reaction solution, and then adding the organic phase to the N-methylpiperazine to continue the reaction, and then adding the reducing powder to obtain the target product. The advantage of this method is that during the preparation process, the intermediate is no needed for separation and purification such as recrystallization. Chloroacetyl chloride and N-methylpiperazine are added dropwise at a lower temperature and the reaction solvent is a single solvent, which greatly shortens the reaction man-hour and simplifies the reaction operation The method has the advantages of mild reaction conditions, simple operation, low production cost, environment friendliness, and is very suitable for industrialized production.
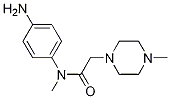
Nintedanib side chain CAS number is 262368-30-9,with full chemical name of N-(4-aminophenyl)-N-methyl-2-(4-methylpiperazin-1-yl)acetamidehe,it is often used for the condensation reaction with the nintedanib intermediate 5. His manufacturing process is relatively simple, there are many patent reports have introduced his synthetic route. In general, there are mainly two synthetic methods. The first one is to obtain the targeted side chain of nintedanib with N-methyl-p-nitroaniline and chloroacetyl chloride as the starting materials ,take two steps reactions. The second, N-methyl-p-nitroaniline and chloroacetic anhydride as raw materials, through two-step reaction to obtain nintedanib side chain. Both of these methods have the problems of complicated operation, long production time and excessive material consumption. A one-pot method is more in line with the actual production, use N-methyl-4-amino-nitrobenzene as a starting material, a dilute solution of chloroacetyl chloride was dropwised, and then incubated for reaction, the reaction was completed, washed with water; organic phase was dropwised into N-Methylpiperazine, and then incubated reaction, the reaction is completed, adding insurance powder and ammonia, heat insulation reaction to obtain the target compound. The advantages of this method such as separation and purification,no recrystallization operation, the temperature is low when dropping chloroacetyl chloride and N- methylpiperazine temperature, the reaction solvent is a single solvent, thus simplifying the reaction operation, greatly reducing the reaction time.
Thera. Category: Anti-cancer
Cas No.: 262368-30-9
Synonym:N-(4-Aminophenyl)-N,4-dimethyl-1-piperazineacetamide;N-[(4-methyl-piperazin-1-yl)methylcarbonyl]-N-methyl-p-phenylendiamine;-N-methyl-2-(4-methylpiperazin-1-yl); N-(4-aminophenyl)-N-methyl-2-(4-methylpiperazin-1-yl)acetamide;
Molecular Weight: 262
Molecular formula:C14H22N4O
Assay: ≥98.%
Appearance: White Crystalline solid
Packing:Export worthy packing
Material Safety Data Sheet:Available on request

.png) Contact Now
Contact Now
