Product Description
Baricitinib intermediate 5 has the chemical name of 4-CHLORO-7-((2-(TRIMETHYLSILYL)ETHOXY)METHYL)-7H-PYRROLO[2,3-D]PYRIMIDINE, we generally refer to baricitinib trimethylsilyl, CAS number is 941685-26-3. In the existing process for preparing Baricitinib trimethylsilyl group, 60% sodium hydrogen is used as a base, and the exothermic heat is intense during the reaction, and a large amount of hydrogen is generated, which is easy to cause combustion and explosion, and sodium. Hydrogen is stored in mineral oil, so the mineral oil introduced in the reaction will affect the quality of the product, and it is difficult to detect in the process. Therefore, 60% sodium hydrogen is not suitable for industrial scale-up production. On the other hand, the solvent used in the prior art process is 1,2-dimethoxyethane (DME), which has a relatively high boiling point and is difficult to remove during the post-treatment, which causes great damage to product quality and subsequent reactions. influences.
One method for preparing Baricitinib trimethylsilyl cas 941685-26-3 is relatively reliable, and the specific procedure is as follows: 328gram potassium t-butoxide and a certain amount of tetrahydrofuran are added to the reaction flask. Between -30℃and -20℃, 300 g of 4-chloro-7H-pyrrolo[2,3-d]pyrimidine in tetrahydrofuran solution was added dropwise to the reaction flask. After the addition was completed, the mixture was stirred at room temperature for 4 to 6 hours. , control the temperature in the reaction flask is not higher than -15℃ ~ -5℃, 390.80g 2- (trimethylsilyl) ethoxymethyl chloride is added to the reaction bottle, after the addition is completed, it is raised to room temperature and Stir at temperature for 3 to 5 hours. After the reaction is completed, the reaction is quenched to neutral with dilute hydrochloric acid, and the tetrahydrofuran in the reaction mixture is concentrated, and then the mixture is stirred, the aqueous phase is separated, and the aqueous phase is extracted once with ethyl acetate. The phase was concentrated, then the ethyl acetate in the reaction mixture was replaced with n-hexane, the reaction solution was cooled, filtered to obtain a wet product, the wet product was crystallized with n-hexane, and dried to obtain baritinib trimethylsilane. Base 534.50g. The purity of the tested product was 99.70%
Thera Category: JAK Inhibitor
Cas No.: 941685-26-3
Synonym: 4-CHLORO-7-((2-(TRIMETHYLSILYL)ETHOXY)METHYL)-7H-PYRROLO[2,3-D]PYRIMIDINE;7-((2-(triMethylsilyl)ethoxy)Methyl)-4-chloro-7H-pyrrolo[2,3-d]pyriMidine;7H-Pyrrolo[2,3-d]pyriMidine, 4-chloro-7-[[2-(triMethylsilyl)ethoxy]Methyl]-
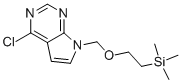
Molecular Formula: C12H18ClN3OSi
Molecular Weight: 283.833
Specifications: Available on request
Packing:Export worthy packing
Material Safety Data Sheet:Available on request
Related intermediates:
1) Pyrazole-4-Boronic Acid Pinacol Ester CAS 269410-08-4
2) 1-Boc-3-(Cyanomethylene)Azetidine CAS 1153949-11-1
3) 4-Chloro-5H-pyrrolo[3,2-d]pyrimidine CAS 84905-80-6
4) 4-Chloro-7-((2-(Trimethylsilyl)ethoxy)Methyl)-7H-Pyrrolo[2,3-d]pyrimidine CAS 941685-26-3
5) (4-Chloro-7H-pyrrolo[2,3-d]pyrimidin-7-yl)methyl Pivalate CAS 1146629-75-5
6) [4-(1H-Pyrazol-4-yl)-7H-Pyrrolo[2,3-d]pyrimidin-7-yl]methyl Pivalate CAS 1146629-77-7
7) Baricitinib (LY3009104, INCB028050) CAS 1187594-09-7 8) 2-(1-(Ethylsulfonyl)azetidin-3-ylidene)acetonitrile CAS 1187595-85-2

.png) Contact Now
Contact Now
