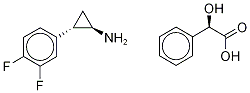Product Description
(1R,2R)-2-(3,4-difluorophenyl)cyclopropanaMine(S)-(carboxylato(phenyl)Methyl)holMiuM cas number 376608-71-8 is an intermediate of Ticagrelor. His synthesis process is:
(1) Dissolve DMA in chloroform, cool down to 0-5 ° C, add trifluoromethanesulfonic anhydride, control the temperature does not exceed 5 ° C, a large amount of white solids precipitated, drip, constant temperature at 0-5 ° C The reaction was carried out for 25 to 35 minutes to obtain a first solution. The 3,4-difluorostyrene and 2,4,6-trimethylpyridine were dissolved in chloroform, and the solution was added dropwise to the first solution, refluxed for 15 to 20 hours, a portion of water was added, and reflux was continued for 15 to 20 hours. Then, it was cooled to room temperature, and the layers were separated to give an organic layer, and then the aqueous layer was extracted with chloroform, and the organic layer extracted with chloroform was combined with the organic layer obtained by layering. Then, it was dried over anhydrous sodium sulfate and subjected to column chromatography to give a pale yellow liquid.
(2) The pale yellow liquid obtained in the first step is dissolved in tetrahydrofuran, and LiHMDS (1M inTHF) is added dropwise at a temperature of -80 to -60℃. After the completion of the dropwise addition, the temperature is kept at -80 to -60℃ for heat preservation. 15 ~ 25min. Then, NBS was added to the solution after the reaction, and the temperature was raised to -5 to 5℃ for 15 to 25 minutes. Further, a sodium hydroxide solution was added, and the reaction was stirred at room temperature for 12 to 24 hours. The product was layered and the aqueous layer was adjusted to pH 3-4 with dilute hydrochloric acid. The aqueous layer was extracted with EtOAc. Column chromatography was then carried out to give an off-white solid.
(3) The white solid obtained in step 2 is dissolved in toluene, a drop of pyridine is added, then thionyl chloride is added, and the temperature is raised to 68-72℃ for 2.5-3.5 hours, and then thionyl chloride is added to continue the heat preservation. The reaction is carried out for 0.8 to 1.2 hours. The reaction solution was evaporated to dryness, and the residue was dissolved in toluene, and then cooled to -5 to 2℃, and then sodium azide, tetrabutylammonium bromide and sodium carbonate were added to carry out the reaction for 1.5 to 2.5 hours. The azide product was diluted with water, layered, and the organic layer was washed with saturated sodium chloride. Next, the toluene was heated to 100 ° C, added dropwise to the organic phase which was layered and washed after azide, and subjected to a rearrangement reaction to form an isocyanate. Then, the resulting isocyanate solution is added dropwise to the hydrochloric acid solution, and the temperature is controlled at 75 to 85℃ for 60 to 70 minutes. After adding water to cool to 25 ° C, stratification was carried out. Further, the aqueous layer in which the salt was dissolved after the hydrolysis under acidic conditions was adjusted to pH 10 to 12 with a sodium hydroxide solution, and extracted with ethyl acetate. After separation, the ethyl acetate layer was washed with water. Then, R-mandelic acid is dissolved in ethyl acetate, and then mixed with the above-mentioned washed ethyl acetate layer, and the temperature is controlled at 16 to 19℃, the reaction is stirred for 2.5 to 3.5 hours, and suction filtration is carried out, and the filter cake is treated with acetic acid. The ester was washed and dried under low temperature to give ticagrelor intermediate (1R,2R)-2-(3,4-difluorophenyl)cyclopropanaMine(S)-(carboxylato(phenyl)Methyl)holMiuM cas number 376608-71-8
Thera. Category: antagonist of the platelet purinergic P2Y12 receptor
Cas No.: 376608-71-8
Synonyms: Ticagrelor InterMediate 4;(1R,2S)-2-(3,4-Difluorophenyl)cyclopropanaMine (2R)-Hydroxy(phenyl)ethanoate;(αR)-α-Hydroxybenzeneacetic Acid coMpd. with (1R,2S)-2-(3,4-Difluorophenyl)cyclopropanaMine; (1R,2R)-2-(3,4-difluorophenyl)cyclopropanamine(S)-(carboxylato(phenyl)methyl)holmium;trans-(1R,2S)-2-(2,3-difluorophenyl)cyclopropylaMine Mandelat;(1R,2S)-2-(3,4-Difluorophenyl)cyclopropanaminium (2R)-hydroxy(phenyl)ethanoate;(alphaR)-alpha-Hydroxybenzeneacetic acid compd. with (1R,2S)-2-(3,4-difluorophenyl)cyclopropanamine;(αR)-(1R,2S)-α-hydroxy-Benzeneacetic acid-coMpd.with 2-(3,4-difluorophenyl)cyclopropanaMine(1:1);
Molecular Formula: C17H17F2NO3
Molecular Weight: 321.3185864
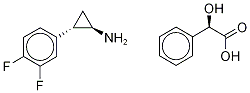
Assay: ≥99%
Packing: Export worthy packing
Material Safety Data Sheet: Available on request
Usage :
Ticagrelor is a reversible antagonist of the platelet purinergic P2Y12 receptor, which is the main receptor responsible for ADP-induced platelet aggregation.
Related intermediate:
1) 274693-27-5 Ticagrelor
2) 4,6-Dichloro-5-nitro-2-(propylthio)pyrimidine 145783-14-8
3) 4,6-Dichloro-2-propylthiopyrimidine-5-amine 145783-15-9
4) (1R,2S)-2-(3,4-difluorophenyl)cyclopropanamine L-Tartaric acid 220352-39-6
5) 1156491-10-9, (1R trans)-2-(3,4-difluorophenyl)cyclopropane amine
6) 376608-74-1, Ethanol,2-[[(3aR,4S,6R,6aS)-6-[[5-aMino-6-chloro-2-(propylthio)-4-pyriMidinyl]aMino]tetrahydro-2,2-diMethyl-4H-cyclopenta-1,3-dioxol-4-yl]oxy]-
7) 376608-71-8, (1R,2S)-2-(3,4-Difluorophenyl)cyclopropanamine (2R)-Hydroxy(phenyl)ethanoate
8) 376608-65-0, 2-((3aR,4S,6R,6aS)-6-amino-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-yloxy)ethanol L-tataric acid
Ticagrelor Impurities:
1)2-((3aS,4R,6S,6aR)-6-amino-2,2-dimethyltetrahydro-3aH-cyclopenta[d][1,3]dioxol-4-yloxy)ethanol (2S,3S)-2,3-dihydroxysuccinate
2) (1R,2R,3S,5R)-3-(7-(((1R,2S)-2-(3,4-difluorophenyl)cyclopropyl)amino)-5-(propylthio)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-3-yl)-5-(2-hydroxyethoxy)cyclopentane-1,2-diol
3) (1R,2R,3S,5R)-3-(7-(((1S,2R)-2-(3,4-difluorophenyl)cyclopropyl)amino)-5-(propylthio)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-3-yl)-5-(2-hydroxyethoxy)cyclopentane-1,2-diol
4) (1S,2S,3R,5S)-3-(7-(((1S,2R)-2-(3,4-difluorophenyl)cyclopropyl)amino)-5-(propylthio)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-3-yl)-5-(2-hydroxyethoxy)cyclopentane-1,2-diol

.png) Contact Now
Contact Now