Product Description
4-Chloro-7-[(4-methylphenyl)sulfonyl]-7H-pyrrolo[2,3-d]pyrimidine is an intermediate of Tofacitinib CAS 479633-63-1, molecular formula: C13H10ClN3O2S, molecular weight:307.76. Regarding the synthesis of 4-Chloro-7-[(4-methylphenyl)sulfonyl]-7H-pyrrolo[2,3-d]pyrimidine, some literature reports use 4,6-dichloro-5-allylpyrimidine as the starting material, The starting material is subjected to an oxidation reaction, and then reacted with ammonia gas to finally close the ring, but the second step of the ammoniated ring in the synthetic route, although the reported yield in the literature is up to 80%, but the yield in many specific experimental attempts is Below 20%, there is a problem that the reaction yield is too low in the route. In addition, the reaction conditions of the synthetic route are complicated, and some expensive reagents are used, and the cost is high, which is disadvantageous for industrial production.
One method is reasonable. It uses 4,6-dichloro-5-allylpyrimidine as a starting material, and undergoes ozone redox reaction, aldol condensation, SN nucleophilic substitution and intramolecular ring closure to obtain4-Chloro-7-[(4-methylphenyl)sulfonyl]-7H-pyrrolo[2,3-d]pyrimidine is an CAS 479633-63-1, the synthetic route is short, the yield is high, and the total yield can reach 59%. The specific implementation process is as follows:
1. To a 50 ml three-necked flask equipped with magnetic stirring, 20 ml of dichloromethane and 5.0 g of 4,6-dichloro-5-allylpyrimidine were added, followed by dimethyl sulfoxide 8 g and triethylamine 5.5 mL. Cool to -40 ℃, pass O3 until the reaction solution turns blue, stop passing O3, use N2 to continue to pass for 30 min, remove O3 from the system. 4.1 g of sodium thiosulfate was added, and the temperature was naturally raised to room temperature, and the starch blue potassium iodide paper was tested to be invariant blue, ensuring that no peroxide was present in the reaction system. After extracting with 60 ml of water, the methylene chloride layer was washed twice with water.
2. Add 5.4 g of the white-like solid obtained in the first step, 8.5 g of triethyl orthoformate and 0.27 g of p-toluenesulfonic acid to a 100 ml three-necked flask equipped with magnetic stirring, add 30 mL of dichloromethane, and heat the system to 40 ℃. , heat preservation reaction for 3h. 4 mL of triethylamine was added to the reaction system, and the reaction system was concentrated, extracted with ethyl acetate, washed twice with water, and then the organic phase was separated, dried over anhydrous sodium sulfate and evaporated to dryness. The ratio of petroleum ether to ethyl acetate was 5:1. Purification by column chromatography gave 4.1 g as a colorless oil.
3. In a 100 ml three-necked flask equipped with magnetic stirring, 5 g of the colorless oily liquid obtained in the step 2 was dissolved in 30 mL of absolute ethanol, and the temperature was raised to 60 ℃, and the mixture was continuously introduced with NH 3 for 3 hours. The reaction mixture was concentrated, and ethyl acetate (30 mL) was evaporated.
4. Dissolve 10.0 g of the product obtained in step 3 in 30 mL of 3M hydrochloric acid, heat to 50 ℃, stir for 4 h, and solid precipitate in the system, suction filtration, and the obtained filter cake is dried to obtain 4.9 g of white solid. The white solid is 4-Chloro-7-[(4-methylphenyl)sulfonyl]-7H-pyrrolo[2,3-d]pyrimidine(an intermediate of Tofacitinib) CAS 479633-63-1.
|
Name:
|
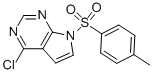
4-Chloro-7-tosyl-7H-pyrrolo[2,3-d]pyrimidine
|
|
CAS:
|
479633-63-1
|
|
Formula:
|
C13H10ClN3O2S
|
|
Molecular Weight:
|
|
|
Molecular structure:
|
|
|
Purity:
|
98%
|
|
Appearance:
|
off-white-white solid
|
Related Intermediates:
1. 3680-69-1 4-Chloropyrrolo[2,3-d]pyrimidine
2. 479633-63-1 4-Chloro-7-tosyl-7H-pyrrolo[2,3-d]pyrimidine
3. 90213-66-4 2, 4-Dichloro-7H-pyrrolo[2,3-d]pyrimidine
4. 1062580-52-2 (3R,4R)-1-BENZYL-N,4-DIMETHYLPIPERIDIN-3-AMINE DIHYDROCHLORIDE
5. 32018-96-5 1-benzyl-4-methylpiperidin-3-one

![Cas 479633-63-1 ,4-Chloro-7-tosyl-7H-pyrrolo[2,3-d]pyrimidine[Tofacitinib Intermediates]](http://img.nbxc.com/product/80/1b/e4/bf71a35089bbc2b3e7ed07cba4.png@4e_360w_360h.src)
.png) Contact Now
Contact Now
