Product Description
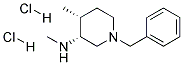
CAS 1062580-52-2,(3R,4R)-1-BENZYL-N,4-DIMETHYLPIPERIDIN-3-AMINE DIHYDROCHLORIDE[Intermediates of Tofacitinib]
Tofacitinib intermediate cas number is 1062580-52-2 ,chemical name is (3R,4R)-1-BENZYL-N,4-DIMETHYLPIPERIDIN-3-AMINE DIHYDROCHLORIDE, molecular formula: C14H24Cl2N2, molecular weight: 291.25976. There are three main methods for the synthesis of tofacitinib piperidamine hydrochloride:
1. Using 3-amino-4-methylpyridine as a starting material, a pyridinium salt is formed with benzyl bromide by an amine transesterification reaction, the salt is first reduced with sodium borohydride, and then reduced to a piperidine ring with platinum oxide. Further, Tofacitinib intermediate cas1062580-52-2 was obtained by reduction of lithium aluminum hydride. The method not only uses platinum, which is a relatively expensive metal reducing agent, but also uses tetrahydrogen lithium aluminum, which undoubtedly increases the safety risk in large industrial production. Moreover, this method does not mention chiral synthesis, and the final product has four isomers, which reduces the optical purity and also reduces the final yield.
2. Using 1-benzyl-4-methyl-1,2,3,6-tetrahydropyridine as a starting material, oxidizing an AryIolefins to a ketone, and then forming an imine with an amine, using an asymmetric reduction imine The amine is formed, the trans isomer is removed by recrystallization to obtain the cis isomer, and finally the chiral resolution is used to obtain the final product (3R,4R)-1-BENZYL-N,4-DIMETHYLPIPERIDIN-3-AMINE DIHYDROCHLORIDE. cas number1062580-52-2,This method not only uses an expensive raw material and a reducing agent, lithium tri-sec-butyl borohydride, but also the reaction is resolved to lower the final yield.
3. 3-Chlorobutyraldehyde is used as raw material to react with sodium cyanide, followed by Leuckart-Wallach reaction and Thorpe-Ziegler reaction, and then reacted with 30% methylamine methanol solution to form enamine, which is subjected to asymmetric catalytic hydrogenation to obtain final product. (3R,4R)-1-BENZYL-N,4-DIMETHYLPIPERIDIN-3-AMINE DIHYDROCHLORIDE. In this method, the Thorpe-Ziegler reaction yield is high, and the asymmetric catalytic hydrogenation reaction does not require chiral resolution of the final product, and the total yield and purity are high, and the by-products are small.
We are Normally supplying related tofacitinib intermediates:
|
Name:
|
(3R,4R)-1-BENZYL-N,4-DIMETHYLPIPERIDIN-3-AMINE DIHYDROCHLORIDE
|
|
CAS:
|
1062580-52-2
|
|
Formula:
|
C14H24Cl2N2
|
|
Molecular Weight:
|
291.25976
|
|
Molecular structure:
|
|
|
Purity:
|
98%
|
|
Appearance:
|
off-white-white solid
|
Related Intermediates:
1. 3680-69-1 4-Chloropyrrolo[2,3-d]pyrimidine
2. 479633-63-1 4-Chloro-7-tosyl-7H-pyrrolo[2,3-d]pyrimidine
3. 90213-66-4 2,4-Dichloro-7H-pyrrolo[2,3-d]pyrimidine
4. 1062580-52-2 (3R,4R)-1-BENZYL-N,4-DIMETHYLPIPERIDIN-3-AMINE DIHYDROCHLORIDE
5. 32018-96-5 1-benzyl-4-methylpiperidin-3-one
6. 477600-75-2, Tofactinib base
7. 540737-29-0, Tofacitnib Citrate
Tofacitinib intermediates, it is our major new project, enable to supplying all kinds of intermediates in high quality

.png) Contact Now
Contact Now
