Product Description
1051375-13-3, Cabotegravir Sodium, GSK1265744 (SodiuM Salt)
l Thera. Category: anticholinergic, used in the treatment of Chronic obstructive pulmonary disease.
l Cas No.: 1051375-13-3
l Synonym: GSK1265744 (sodiuM salt);Cabotegravir Sodium
l Molecular Formula: C19H17F2N3NaO5+
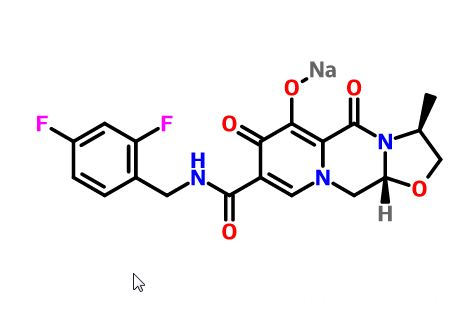
l Molecular Weight: 428.34
l Assay: ≥98.%
l Packing: Export worthy packing
l Material Safety Data Sheet: Available on request
l Usage: Cabotegravir Sodium
Background
GSK744 (S/GSK1265744) is a potential inhibitor of HIV integrase with IC50 value of 3 nM [1].
HIV integrase is an enzyme produced by HIV virus that enables its genetic material to be integrated into the infected cell DNA. It is reported that HIV intergrase inhibitor plays an important role in halting HIV progression [2].
GSK744 (S/GSK1265744) is a potent HIV integrase inhibitor. Using resistance passage experiments, integrase enzyme assays, and cellular assays with site-directed molecular (SDM) HIV clones resistant to other classes of anti-HIV-1 agents and earlier integrase strand transfer inhibitors, results showed that GSK1265744 efficiently inhibited HIV replication through inhibiting HIV integrase [1].
In female pigtail macaques model that intravaginal inoculated simian/human immunodeficiency virus twice a week for up to 11 weeks, GSK744 injection prevented the macaques from being infected by virus while placebo controls were infected after a 4 median vaginal challenges with SHIV which reminded that GSK744 may be a potential preexposure prophylaxis drug for prevention via inhibiting HIV integrase [3] [2].
Many clinical trials have been conducted to show that GSK744 can efficiently protected healthy subjects from HIV infection [4-6].
References:
[1]. Yoshinaga, T., et al., Antiviral characteristics of GSK1265744, an HIV integrase inhibitor dosed orally or by long-acting injection. Antimicrob Agents Chemother, 2015. 59(1): p. 397-406.
[2]. Andrews, C.D., et al., Long-acting integrase inhibitor protects macaques from intrarectal simian/human immunodeficiency virus. Science, 2014. 343(6175): p. 1151-4.
[3]. Radzio, J., et al., The long-acting integrase inhibitor GSK744 protects macaques from repeated intravaginal SHIV challenge. Sci Transl Med, 2015. 7(270): p. 270ra5.
[4]. Ford, S.L., et al., Lack of pharmacokinetic interaction between rilpivirine and integrase inhibitors dolutegravir and GSK1265744. Antimicrob Agents Chemother, 2013. 57(11): p. 5472-7.
[5]. Spreen, W., et al., Pharmacokinetics, safety, and tolerability with repeat doses of GSK1265744 and rilpivirine (TMC278) long-acting nanosuspensions in healthy adults. J Acquir Immune Defic Syndr, 2014. 67(5): p. 487-92.
[6]. Spreen, W.R., D.A. Margolis, and J.C. Pottage, Jr., Long-acting injectable antiretrovirals for HIV treatment and prevention. Curr Opin HIV AIDS, 2013. 8(6): p. 565-71.
We are availabe for several Intermediates of Cabotegravir:
1) 1335210-24-6, Oxazolo[3,2-a]pyrido[1,2-d]pyrazine-8-carboxylic acid, 2,3,5,7,11,11a-hexahydro-6-methoxy-3-methyl-5,7-dioxo-, (3S,11aR)
2) 1335210-25-7, (3S,11aR)-N-(2,4-difluorobenzyl)-6-methoxy-3-methyl-5,7-dioxo-2,3,5,7,11,11a-hexahydrooxazolo[3,2-d]pyrido[1,2-a]pyrazine-8-carboxamide
3) 1051375-10-0, Cabotegravir Free Acid
4) 1051375-13-3, Cabotegravir Sodium


.png) Contact Now
Contact Now
